An abiding quest to understand pain
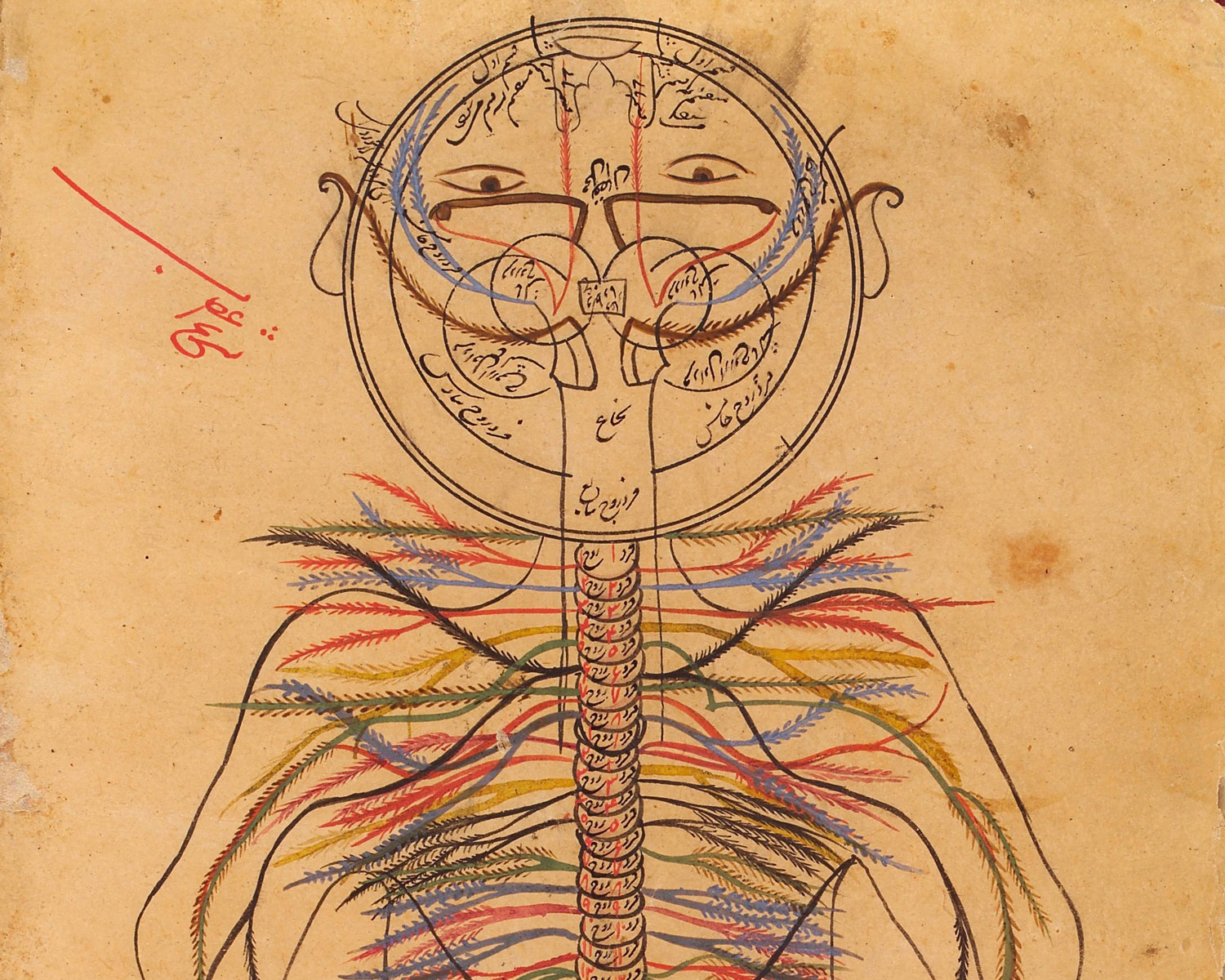
Physicians have long disagreed on the exact nature of pain. Galen of Pergamon, an influential ancient Roman surgeon and philosopher, believed that only injuries caused pain. The great Persian physician Avicenna, the author of The Canon of Medicine, an 11th-century book that influenced pain medicine for centuries, mapped the peripheral nervous system (shown). He described pain as a specific sensation, separate from touch or temperature — but no one understood how our bodies sense it.
Credit: Wellcome Coll., CC BY 4.0
Pain pathways emerge
Rene Descartes, the great 17th-century philosopher and scientist, explained pain sensation as particles of heat moving through a ‘pain pathway,’ a phrase still used today. In the early 1900s, the English neurophysiologist Sir Charles Sherrington (shown) traced the neural circuitry of reflexes, including the withdrawal reflex, which makes us snatch back our hand from a hot stove. He proposed that pain comes from tissue injury and that receptors called ‘nociceptors’ specifically sense pain.
Credit: NLM/SPL
New routes to pain relief
Healthy pain alerts us to danger, disease or injury, but chronic pain causes great suffering. To relieve pain, Western doctors moved from laudanum — a mixture of opium and sherry — in the 1700s, to ether in the 1800s, then heroin and morphine in the 1900s. Along the way, in 1899 Bayer began selling aspirin, a synthetic chemical related to a compound from willow bark, a popular folk remedy. But even today chronic pain affects 20 percent of the population, and there is no reliable way to treat it.
Credit: Sebastian Kaulitzki/SPL
David Julius discovers the first nociceptor
Capsaicin, the chemical that makes peppers painfully hot, activates two types of nerve fibers. In a pivotal 1997 study, Julius’s team moved genes from those nerve fibers into skin cells that fluoresce only when responding to capsaicin. This revealed the capsaicin receptor — a protein called TRPV1. Pouring hot solutions on the cells also made them fluoresce. This identified TRPV1 as the first known nociceptor, and as the protein that lets us sense heat.
Watch David Julius discuss how we sense pain. Credit: Steve Babuljak
The false hope of prescription opioids
Opioids kill severe and chronic pain by binding to neural receptors in the brain or spinal cord. In the late 1990s, pharmaceutical companies that make opioids offered safety assurances that caused opioid prescriptions to climb (yellow). A wave of addiction and overdose deaths (orange) followed. Changing guidelines and public perception since 2011 have lowered the volume of opioids prescribed, but prescription opioid overdoses still kill about 15,000 Americans a year. An equally effective, yet nonaddictive, analgesic is desperately needed.
Credit: Alisdair Macdonald
Can machines offer objective diagnosis?
Overdiagnosing pain can lead to addiction, while underdiagnosing it can lead to chronic pain. Last year MIT researchers reported a wearable device that measures pain objectively. The device combines functional near infrared spectroscopy (fNIRS), which measures neuronal activity in a pain-sensing brain region, and machine learning that improves image processing and analysis. This and other AI tools could provide accurate pain assessment, even on unconscious or uncommunicative patients, which could lead to better clinical outcomes.
Courtesy of: D. Lopez-Martinez/Harvard-MIT and MIT Media Lab.; K. Peng, Harvard Med. School and CHUM Res.; A. Lee and D. Borsook/Harvard Med. School, R. Picard/MIT Media Lab
Can a single pill cure all pain?
No magic bullet for pain is possible because there are too many different types of pain, from bladder pain to a migraine, Julius says. But pain sensing research offers insights that could lead to rational design of pain medications that target specific nociceptors. Such drugs could block pathological pain, while allowing pain signals that protect us from injury, such as sensing the heat of a flame.
Credit: Ralf Hiemisch/Getty